Insight into C-C bond hydrolases
The cleavage of C-C bonds is a challenging, but necessary reaction.
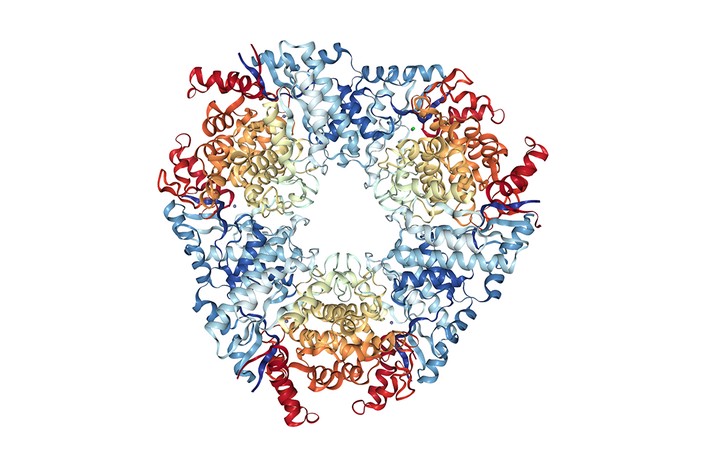
The enzymatic hydrolysis of C-C bonds presents a unique challenge as the scissile bond must be activated prior to bond cleavage. MCP hydrolases, involved in the catabolism of aromatic compounds and steroids, accomplish this activation by first catalyzing an enol-keto tautomerization of the substrate, a β,γ-unsaturated δ-diketone. Most MCP hydrolases are serine-dependent enzymes, in which a strained substrate is thought to deprotonate the catalytic serine, simultaneously completing the ketonization and activating the serinate nucleophile (Ruzzini, et al. 2013). We recently discovered a family of Zn(II)-dependent MCP hydrolases that catalyzes the same enol-keto tautomerization as the serine-dependent enzymes but in which hydrolysis appears to proceed via a gem-diol intermediate instead of an acyl-enzyme intermediate (Kuatsjah et al., 2018; Nerdinger et al. 2018; Kuatsjah et al. 2017). These studies provide new insights into C-C bond hydrolases as well as how the catalytic machinery of different superfamilies has evolved to catalyze a range of reactions (Ruzzini et al, 2019).